URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: High-speed camera snaps bio-switch in action
News
News from the DESY research centre
High-speed camera snaps bio-switch in action
With a powerful X-ray camera, scientists have watched a genetic switch at work for the first time. The study led by Yun-Xing Wang from the National Cancer Institute of the U.S. reveals the ultrafast dynamics of a riboswitch, a gene regulator that can switch individual genes on and off. The innovative technique used for this investigation opens up a completely new avenue for studying numerous fundamental biochemical reactions, as the team reports in a fast-track publication in the journal Nature.

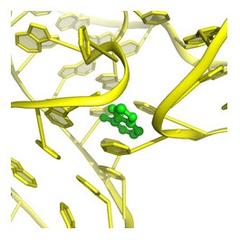
The researchers studied a riboswitch from the bacterium Vibrio vulnificus, a close relative of the cholera germ. It can cause infections that are especially hard to treat and often fatal. The switch is activated by a signal molecule, known as a “ligand”, in this case adenine. Upon activation the riboswitch changes its form. As a result, the gene where the switch is located will no longer be read out and thus becomes deactivated. Riboswitches are present in bacteria and fungi, but not in mammals including humans. Targeting the genetic switches in bacteria might offer a powerful weapon in the fight against diseases.
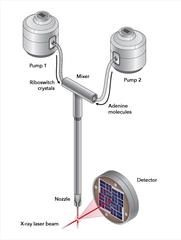
In a new device developed by Chapman's group, the aptamer crystals can be mixed with a solution of the signal molecule, adenine. The adenine diffuses into the crystal and “pushes the button” in the riboswitch, starting the biochemical interaction. The adenine soaked nanocrystals were injected into the beam of the X-ray laser, while a delay line allowed to freeze-frame the biochemical reaction a short time after it began.
This way, the scientists discovered an intermediate state of the riboswitch that has never been seen before and that likely only exists for milliseconds in a living organism. “Previous experiments at SLAC’s X-ray laser have studied biological reactions like photosynthesis that are triggered by light. But this is the first to observe one that is triggered by the chemical interaction of two biomolecules in real time and at the atomic scale,” says Wang. “This really demonstrates the unique capability that X-ray free-electron lasers offer that no current technology, or any other technology on the horizon, can do. It’s like you have a camera with a very fast shutter speed, so you can catch every move of the biomolecules in action.”
The study proves the concept of “mix-and-inject” crystallography to investigate biochemical reactions, explains Chapman, who is also a professor at the University of Hamburg and a member of the Hamburg Centre for Ultrafast Imaging (CUI).
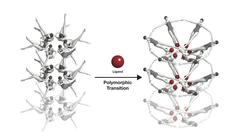
The riboswitche's conformational change, symbolised with swimmers: When a riboswitch meets a ligand (red), it changes its form. Credit: Joseph Meyer, National Cancer Institute
“Almost all proteins, RNAs, and DNAs interact with ligands or substrates and undergo certain conformation changes when they react. The ability to visualise these changes is critical to understanding how biomacromolecules perform their functions,” says Wang. “In the past, people studied these kinds of reactions indirectly or in very limited cases where the conformation changes were very small without cracking crystals. With our method, we are now able to directly visualise the structures and changes in real time in a much broader range of biochemical interactions and reactions.”
In addition to the National Cancer Institute and other National Institutes of Health, SLAC and DESY, the Arizona State University, the Johns Hopkins University, the Hauptmann-Woodward Medical Research Institute, the Structural Biology Center and the Argonne National Laboratory were involved in this research.
Reference:
Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography; J.R. Stagno, Y. Liu, Y.R. Bhandari, C.E. Conrad, S. Panja, M. Swain, L. Fan, G. Nelson, C. Li, D.R. Wendel, T.A. White, J.D. Coe, M.O. Wiedorn, J. Knoska, D. Oberthuer, R.A. Tuckey, P. Yu, M. Dyba, S.G. Tarasov, U. Weierstall, T.D. Grant, C.D. Schwieters, J. Zhang, A.R. Ferré-D’Amaré, P. Fromme, D.E. Draper, M. Liang, M.S. Hunter, S. Boutet, K. Tan, X. Zuo, X. Ji, A. Barty, N.A. Zatsepin, H. N. Chapman, J.C.H. Spence, S. A. Woodson & Y.-X. Wang; Nature, 2016; DOI: 10.1038/nature20599
Further reading:
• Research summary from the National Cancer Institute
• News and video from SLAC National Accelerator Center