URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Scientists X-ray individual nanoparticles for catalyst research
News
News from the DESY research centre
Scientists X-ray individual nanoparticles for catalyst research
A team of international scientists has studied individual nanoparticles made from an alloy of platinum and rhodium, two catalytic materials, under conditions encountered in technical applications. The analysis using X-rays and a scanning electron microscope shows how these two metals respond to different external conditions and accumulate on the surface of the nanoparticle in the process. The team headed by Hoydoo You from the Argonne National Laboratory in the USA is presenting its study in the journal Physical Review Letters.
For their study, the scientists at DESY NanoLab came up with a way of manufacturing alloy nanoparticles of a size suitable for making X-ray measurements. To do so, platinum was first deposited on a substrate in a vacuum, using heat treatment to adjust their size, after which the platinum was coated with rhodium at high temperatures, in order to achieve the optimum level of mixing. Afterwards a nanoparticle was selected in the scanning electron microscope and individual markers were applied. In the X-ray experiment, the sample was then exposed to a range of different temperatures and atmospheres of varying gaseous compositions, while the previously marked particle was studied in the X-ray beam.
The scientists used X-rays from the Advanced Photon Source in Argonne to determine the spatial distribution of the displacements in the crystal lattice, making use of the fact that the atoms of the two elements differ slightly in size. This means they can switch positions within the crystal lattice and thereby migrate through the particle – driven by external factors such as the temperature or gas composition. The local crystal lattice therefore shifts slightly, and the measured displacement can be translated into the local change in the concentration of the rhodium atoms.
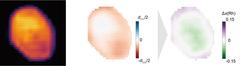
Shape of the alloy nanoparticle (electron density), lattice displacements and resulting relative rhodium distribution in alloy nanoparticle at a temperature of 700°C (left to right). Regions with higher rhodium concentrations are shown in purple, regions with a reduced concentration are green. Image: Argonne National Laboratory and Tohoku University, Tomoya Kawaguchi.
Being a very active catalyst, rhodium is of considerable interest for many reactions. However when it becomes fully oxidised on the surface of the catalyst, it becomes inactive and is no longer available for catalysis. This is why catalysts should ideally be tailored to fit the specific application, depending on the prevailing conditions in the environment.
“Under reductive conditions, for example in a hydrogen-rich atmosphere, the rhodium atoms return from the oxidised state back into the crystal lattice,” as the principal author Tomoya Kawaguchi from the Argonne National Laboratory and Tohoku University in Japan explains.
“We are now trying to understand how such a nanoparticle can best be kept active,” says Keller. “The method we have developed for marking them allows us for the first time to characterise individual nanoparticles and to proceed to analyse them further at DESY NanoLab, instead of studying a large number of particles and looking at the mean, as is normally the case. This means that systematic effects can be studied directly.”
The experiments were conducted by the Argonne National Laboratory, Tohoku University, the University of Hamburg, the National Research Nuclear University MEPhI in Moscow and by DESY.
Reference:
Tomoya Kawaguchi, Thomas F. Keller, Henning Runge, Luca Gelisio, Christoph Seitz, Young Y. Kim, Evan R. Maxey, Wonsuk Cha, Andrew Ulvestad, Stephan O. Hruszkewycz, Ross Harder, Ivan A. Vartanyants, Andreas Stierle, and Hoydoo You; Gas-induced segregation in Pt-Rh alloy nanoparticles observed by in situ Bragg coherent diffraction imaging; Physical Review Letters, (2019); DOI: 10.1103/PhysRevLett.123.246001



