URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Vesicle Origami
News
News from the DESY research centre
Vesicle Origami
For the first time, scientists have observed a phosphorus-containing lipid molecule that assembles by itself to form cubes. Research carried out at facilities including DESY has shown that the unusual shape is due to special bonds in the synthetic molecule, a particular phospholipid. Phospholipids play an important role in living organisms, forming membranes, among other things. The new findings enhance the understanding of the forces acting within biological membranes and could open up new pathways in medicine. The researchers headed by Andreas Zumbühl from the University of Fribourg in Switzerland present their results in the journal Angewandte Chemie.

Artist's impression of the observed phospholipid cubes. The molecules are so closely packed that the membrane can scarcely be bent, resulting in the cuboid shape. Credit: Moser Graphic Design http://moser.ch
Normally, such vesicles are spherical in shape, to minimise the surface tension. However, the 1,2-diamidophospholipid now analysed by the scientists produces cuboid vesicles at room temperature. This is because this phospholipid forms very close-packed and therefore very stiff layers, which are very difficult to bend, thanks to special bonds, known as hydrogen bonds, which minimise the distance between the molecules. When it assembles as a three-dimensional structure, the membrane continues to favour flat surfaces and structures having as few edges as possible, conditions that are satisfied by a cube.
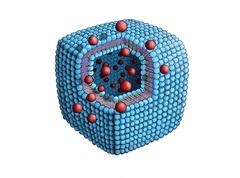
Phospholipid cubes like these could one day be used for targeted drug delivery. The edges of the cube are its weakest point, so that it can be opened by shaking or applying larger forces, releasing the enclosed substance when and where it is needed. Credit: Moser Graphic Design http://moser.ch
For the team of research scientists, the phospholipid examined is most of all an important step on the way to a greater objective: “We would like to understand what forces are acting in the membrane, so that we can later deliberately influence these. This would allow us to use phospholipids as a kind of building material, so as to construct specific structures on a cellular level,” says Zumbühl. To understand the precise details of the phospholipids, the scientists synthesise certain molecules, modifying their structure and properties slightly each time, in order to see what effect this has. Because a small change in the structure of a phospholipid can have a large effect.
The beamline P08 at DESY's X-ray source PETRA III had to be specially equipped for these kind of structural investigations at the boundary between air and water. „Thanks to the optimisation of our set-up and the exact control of the temperatures and pressures acting on the membranes, even the surface pressure in an individual layer of the 1,2-diamidophospholipid could be determined,“ explains beamline scientist Olof Gutowski from DESY, who made these measurements possible. The result surprised the scientists: “For 30 years, it has generally been assumed that the pressure in a biological membrane must be relatively high, around 30 Millinewton per metre,” says Zumbühl. “In the membrane we studied, however, the pressure must be considerably lower, around 5 to 10 Millinewton per metre. This calls into question the long-standing rule of thumb.”
Scientists from the Paul Scherrer Institute in Switzerland and the Max Planck Institute of Colloids and Interfaces in Potsdam were also involved in the study.
Reference:
Vesicle Origami: Cuboid Phospholipid Vesicles Formed by Template-Free Self-Assembly; Frederik Neuhaus, Dennis Mueller, Radu Tanasescu, Sandor Balog, Takashi Ishikawa, Gerald Brezesinski, and Andreas Zumbuehl; Angewandte Chemie, 2017; DOI: 10.1002/anie.201701634



