URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: X-rays show self-organisation of nanocrystals live
News
News from the DESY research centre
X-rays show self-organisation of nanocrystals live
Using X-rays, a DESY research team has watched live how nanocrystals organise themselves into a highly ordered layer. The study helps to better understand the self-organisation of nanoparticles, which is important for many technical applications, for example in electronics and optoelectronics, as the scientists write in the journal Small.
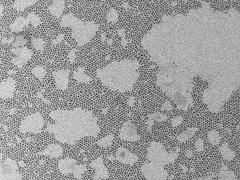
Electron micrograph of unordered lead sulfide nanocrystals. Credit: DESY, Irina Lokteva

For the time-resolved investigation, the nanocrystals were suspended in the organic solvent heptane. 25 microlitres (millionths of a litre) of the suspension were dropped into a sample holder 7 millimetres high and just 0.5 millimetres wide, held in a vacuum. As the solvent evaporated slowly during about two hours, the scientists could watch how a 3D highly ordered layer of nanocrystals formed at the walls of the sample holder from the top to the bottom.
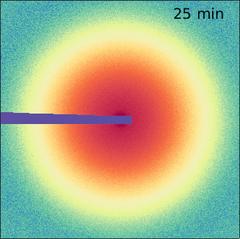
“Suddenly, the entire layer jumps into a different structure,” says Lokteva. “Since this is accompanied by a change in volume, cracks appear in the layer.” This effect, which the team observed live for the first time, can either be used specifically or avoided in order to produce layers with desired properties. “With the right framework conditions for self-organization, the final structure can be influenced according to the requirements of the application,” explains Lokteva.
The observation of the lead sulfide model system also yields important insights for other materials. “Numerous applications of nanoparticles require ordered films,” says Lokteva. The research results are a step towards better understanding both the self-organisation of nanoparticles and the interaction of the oleic acid ligands with the organic solvent heptane.

The X-ray diffraction patterns of the unordered suspension (left) and of the nanocrystal layer with hcp structure (centre) and the final bcc structure (right). Credit: DESY, Irina Lokteva
Monitoring Nanocrystal Self-Assembly in Real Time Using In Situ Small-Angle X-Ray Scattering; Irina Lokteva, Michael Koof, Michael Walther, Gerhard Grübel und Felix Lehmkühler; Small, 2019; DOI: 10.1002/smll.201900438



