URL: https://www.desy.de/news/news_search/index_eng.html
Breadcrumb Navigation
DESY News: Astonishing diversity: Semiconductor nanoparticles form numerous structures
News
News from the DESY research centre
Astonishing diversity: Semiconductor nanoparticles form numerous structures
The structure adopted by lead sulphide nanoparticles changes surprisingly often as they assemble to form ordered superlattices. This is revealed by an experimental study that has been conducted at DESY’s X-ray source PETRA III. A team led by the DESY scientists Irina Lokteva and Felix Lehmkühler, from the Coherent X-ray Scattering group headed by Gerhard Grübel, has observed the self-organisation of these semiconductor nanoparticles in real time. The results have been published in the journal Chemistry of Materials. The study helps to better understand the self-assembly of nanoparticles, which can lead to significantly different structures.
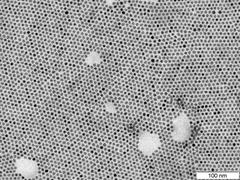
The lead sulphide nanoparticles, which are about eight nanometres (millionths of a millimetre) in size, initially arrange themselves into a layer with hexagonal symmetry. Credit: University of Hamburg, Stefan Werner
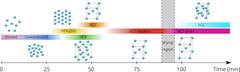
To their surprise, the structure adopted by the particles changed several times during the process. “First we see the nanoparticles forming a hexagonal symmetry, which leads to a nanoparticle solid having a hexagonal lattice structure,” Lokteva reports. “But then the superlattice suddenly changes, and displays a cubic symmetry. As it continues to dry, the structure makes two more transitions, becoming a superlattice with tetragonal symmetry and finally one with a different cubic symmetry.” This sequence has been never revealed before in such detail.
The team suggests that the hexagonal structure (hexagonal close-packed, HCP) persists for as long as the surface of the particles is swollen by the solvent. Once the film dries a little bit, its internal structure changes to a cubic symmetry (body-centred cubic, BCC). However, residues of the solvent still remain between the individual nanoparticles inside the film. As this evaporates, the structure changes two more times (body-centred tetragonal BCT and face-centred cubic FCC).
“Our research indicates that the final structure of the superlattice also depends on whether the individual nanoparticles are surrounded by many or few oleic acid molecules,” reports Lokteva. “In an earlier study, we obtained films with a BCC/BCT crystal structure when the ligand density was high. Here, we specifically looked at nanoparticles with a low ligand density, and this led to an FCC structure. So when using nanoparticles, the ligand density ought to be determined, which is not a standard practice at the moment,” explains the DESY scientist.
These observations are also important when it comes to other materials, the team points out. “Lead sulphide is an interesting model system that helps us to better understand the general mechanisms by which nanoparticles self-assemble,” Lokteva explains. “Nature can provide nanostructures with various interesting properties via the phenomenon of self-assembly, and we now have the tools to look over nature's shoulder as it constructs these structures.”
Reference:
Real-Time X-ray Scattering Discovers Rich Phase Behavior in PbS Nanocrystal Superlattices during In Situ Assembly; Irina Lokteva, Michael Dartsch, Francesco Dallari, Fabian Westermeier, Michael Walther, Gerhard Grübel, and Felix Lehmkühler; Chemistry of Materials, 2021; DOI: 10.1021/acs.chemmater.1c02159



