Proteins in a crowd: European XFEL looks inside cells
Researchers observe in detail how the vital iron protein ferritin makes its way in highly dense environments
Inside biological cells, there is a dense crowd where millions of proteins move side by side, bump into each other or temporarily accumulate. At the same time, these proteins often have to fulfil important tasks at short notice. How exactly the proteins move in this confined space has been difficult to track until now. An international research team led by Anita Girelli and Fivos Perakis, both from Stockholm University, has now used the European XFEL X-ray laser in Schenefeld near Hamburg to take a closer look at these movements—and discovered a surprising pattern.

Molecules in a “cage”
The experiments focussed on ferritin, a spherical protein that stores iron and is found in almost all living organisms. When it is examined in high concentrations, it exhibits unusual behaviour: Instead of moving evenly and randomly—as in classical Brownian motion—ferritin repeatedly finds itself in a kind of molecular cage: surrounded by neighbouring proteins, for short times it is blocked before it is freed again and can move on.
Snapshots every microsecond
To visualise these tiny movements, the team used a new technique: megahertz X-ray photon correlation spectroscopy (MHz-XPCS). “With the extremely fast X-ray flashes from European XFEL, we are able to measure how the proteins move in only a millionth of a second,” explains Johannes Möller, scientist at the ‘Materials Imaging and Dynamics’ (MID) instrument at European XFEL. The research is closing a gap between established methods such as light scattering or nuclear magnetic resonance, which can also measure protein movements, but not with such precision and speed.
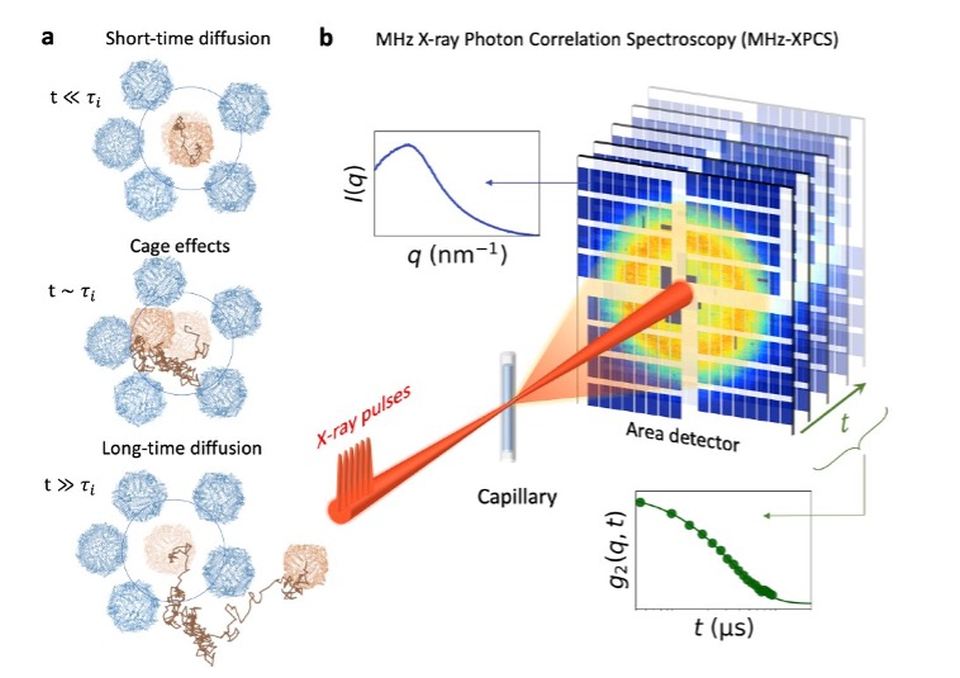
Unexpected insights—practical consequences
The observations show that the denser the environment, the more pronounced the cage phenomenon becomes. “The proteins do not simply move more slowly, but in a complex way that is unusually restricted,” says Anita Girelli, leader of this study. “These findings are not only relevant for basic research. They could also help to develop new biomedical applications,” adds her colleague Fivos Perakis.
“Thanks to its unique MHz repetition rate, the European XFEL is currently unmatched worldwide for experiments that let us watch nanoscale dynamics and motion in remarkable detail, revealing surprises as the unexpected behaviour of these protein complexes in solution,” says DESY scientist Felix Lehmkühler, a co-author of the study.
Ferritin is already being investigated in drug delivery where drugs can be packaged inside the protein to delay their release in the body. How quickly the proteins spread and diffuse directly influences their effectiveness. Other applications for ferritin are also being discussed, for instance as a contrast agent for magnetic resonance imaging (MRI) or as building blocks for nanomaterials.
“With the European XFEL, we were able to track the collective movements of proteins more precisely than ever before,” says Girelli. This not only helps to better understand the fundamentals of biological processes—it also opens up new perspectives for medical applications.
The study was carried out as part of a long-term project at the MID instrument. Participants included the Universities of Siegen and Tübingen, the Technical University of Dortmund, ESRF—The European Synchrotron, and the Institute Laue-Langevin (ILL), both in Grenoble, and the Deutsches Elektronen-Synchrotron DESY in Hamburg.
Reference
Coherent X-rays reveal anomalous molecular diffusion and cage effects in crowded protein solutions. Girelli, A., Bin, M., Filianina, M. et al. Nat Communications (2025). https://doi.org/10.1038/s41467-025-66972-6
